We frequently hear that regenerative thermal oxidizers (RTOs) are very expensive to operate. After all, they do consume natural gas and normally operate at 1500°F or higher. This can add up to very large natural gas or propane annual operating costs.
But what are the realities? To understand, let’s consider some typical cases.
How an RTO Works
Before we do that, let’s look at how an RTO works. An RTO is a simple VOC incineration machine that utilizes a well-known regenerative process that stores and returns heat in ceramic heat exchange beds. As shown on the simplified diagram below, the back-and-forth cycling of the gas stream allows most of the energy in the combustion zone to stay within the machine so that the net temperature increase from inlet to outlet is minimized. With this, the energy consumption is also minimized even though the contaminated gas is treated at more than 1500°F to destroy the pollutants.
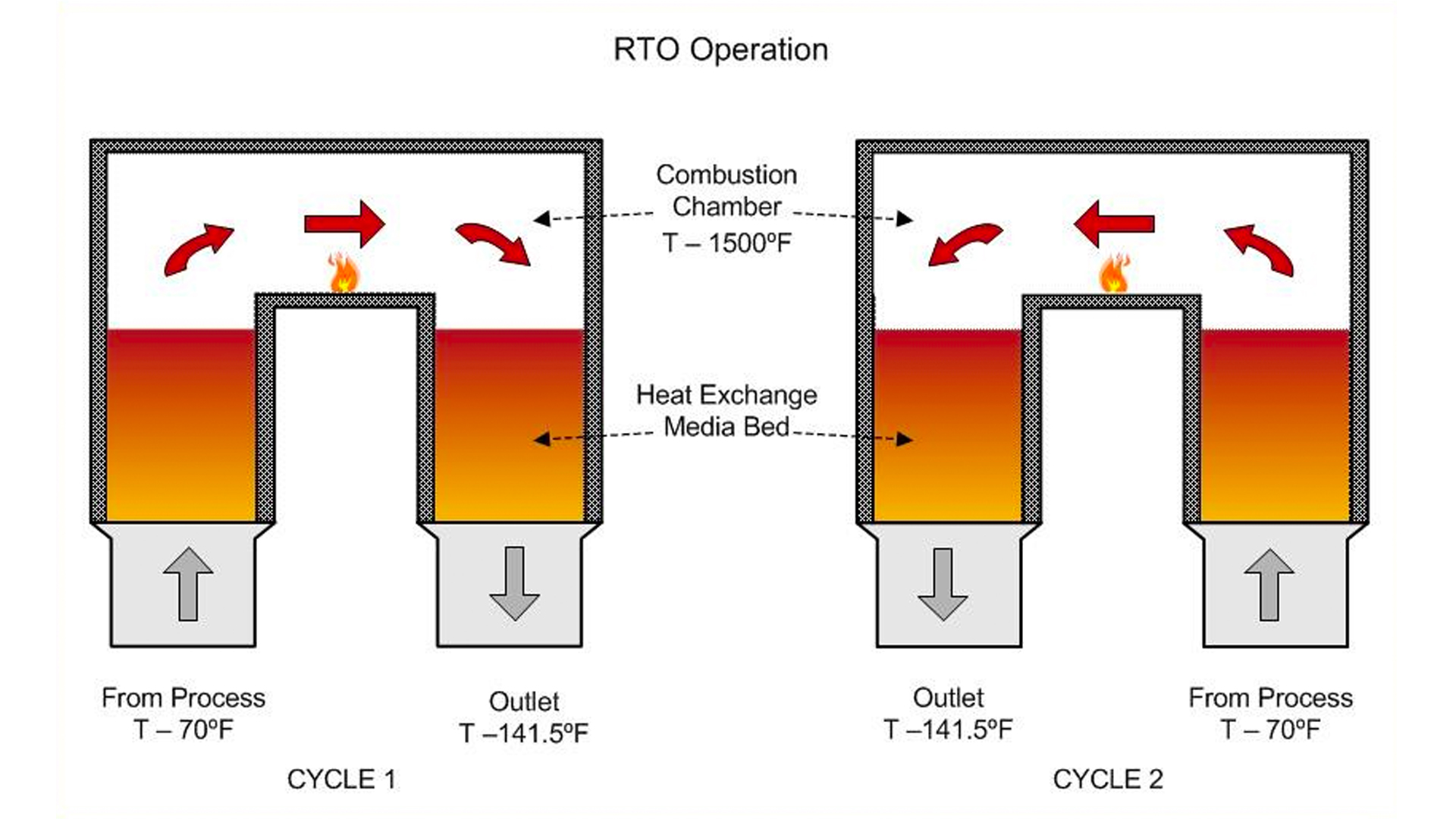
Of course, how much heat is retained and returned within the RTO is a very important factor. A term that is used to characterize this effect is thermal efficiency or TE. The higher the value of TE, the more energy efficient a given RTO design is. For most RTOs the operating value for TE ranges from 90% to 97%. A good way to think about the meaning of TE is this: TE is the ratio of the energy recovered to the energy added. For example, in the diagram above, the TE is 94% and can be calculated by dividing the the energy recovered (1500°F to 150°F) by the energy added (70°F to 1500°F); i.e., 1350°F /1430°F = 0.94.
The missing factor in all of this is the energy added by the combustion of the volatile organic compounds (VOCs). Virtually all VOCs yield heat when they are burned. The same is true for carbon monoxide. To understand how much the VOC and/or CO contribution can make to the operating cost of an RTO we will look at some examples.
First a base case. No VOCs or CO. Just ambient air.
Assuming 100,000 scfm (440,000 lb/h) of air at ambient temperature of 70°F and a TE of 95% for the RTO we calculate an air temperature increase of 71.5°F. To calculate the amount of natural gas required to heat the air one can use a simple equation;
Q = mCpT
Where
Q = total heat required (BTU/h)
m = mass of air being treated (440,000 lb/h)
Cp = heat capacity of air (0.25 BTU/lb°F)
T = temperature increase (71.5 °F)
From this we calculate, Q = 7,865,000 BTU per hour. In terms of natural gas required, this is 78.65 therm/h and at $0.40/therm (2019 approx. rates) the hourly cost is $31.46. On a yearly basis this is $275,589, a very significant addition to a plant’s overhead.
Second Case. Add VOCs.
Suppose the gas stream has 500 ppm of VOC measured as propane. 500 ppm is, in fact, a very dilute amount representing only 0.05% of the gas volume. Yet, it still adds a significant heat boost to the RTO operation as it burns in the combustion chamber. Assuming it has the same heat content as propane, 18,000 BTU per pound, the 500 ppm of VOC adds 6,326,424 BTU/h to the energy balance of the RTO and results in a reduction in the natural gas consumed to only 1,538,575 BTU/h (7,865,000 – 6,326,424). The annual gas bill now is only $53,918, translating to a lower yearly operating cost difference of $221,671.
Further, if the VOC concentration is 600 ppm the natural gas consumption falls to nearly zero and the RTO is approaching self-sustaining operation.
While this analysis does not include the relatively minor additions of radiant heat loss and burner combustion air it clearly shows how just a small amount of VOC can dramatically reduce the fuel requirements and operating cost of an RTO system. The chart below shows this in more detail.
Finally, there is even more good news on this subject. A TE of 95% is good but a TE of >96% is quite possible with today’s modern RTOs. The 1% difference between 95% and 96% may not sound like much but it means 20% less fuel required. And, as illustrated above, 20% can make a significant improvement in operating expenses.
Note that there is the catalytic approach, another fuel saver, but we’ll leave that for another technical blog.
For more information, contact us.